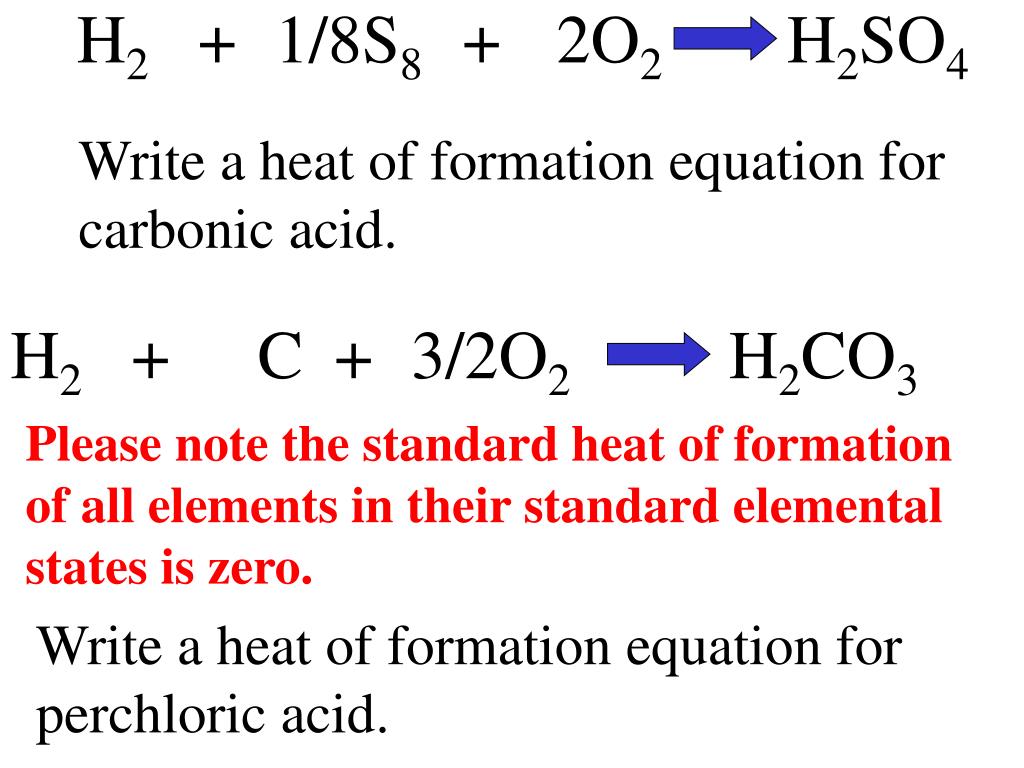
Standard Heat Of Reaction Using Enthalpies Of Formation . the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. to find the δh reaction o, use the formula for the standard enthalpy change of formation: So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. \[\delta h_{reaction}^o = \sum {\delta. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and.
from www.slideserve.com
193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. \[\delta h_{reaction}^o = \sum {\delta. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. to find the δh reaction o, use the formula for the standard enthalpy change of formation: a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as. the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm.
PPT Standard Heats of Reaction PowerPoint Presentation, free download
Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. to find the δh reaction o, use the formula for the standard enthalpy change of formation: \[\delta h_{reaction}^o = \sum {\delta. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Heat Of Reaction Using Enthalpies Of Formation the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. \[\delta h_{reaction}^o = \sum {\delta. the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. a standard enthalpy. Standard Heat Of Reaction Using Enthalpies Of Formation.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Reaction Using Enthalpies Of Formation \[\delta h_{reaction}^o = \sum {\delta. So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. the standard state for measuring and reporting enthalpies. Standard Heat Of Reaction Using Enthalpies Of Formation.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Heat Of Reaction Using Enthalpies Of Formation the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. a standard enthalpy of formation δ h f ° δ h f °. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. So at this. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. 193 rows the standard enthalpy change of any reaction can be calculated from. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Heat Of Reaction Using Enthalpies Of Formation \[\delta h_{reaction}^o = \sum {\delta. the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as. 193 rows the standard enthalpy change of any reaction can be calculated from the. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Heat Of Reaction Using Enthalpies Of Formation \[\delta h_{reaction}^o = \sum {\delta. So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows the standard enthalpy change of any reaction can be calculated. Standard Heat Of Reaction Using Enthalpies Of Formation.
From fity.club
Enthalpy Equation Delta H Standard Heat Of Reaction Using Enthalpies Of Formation to find the δh reaction o, use the formula for the standard enthalpy change of formation: a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. \[\delta h_{reaction}^o = \sum {\delta. a standard enthalpy of formation δ h f ° δ h f ° is an. Standard Heat Of Reaction Using Enthalpies Of Formation.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows. Standard Heat Of Reaction Using Enthalpies Of Formation.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Heat Of Reaction Using Enthalpies Of Formation So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Heat Of Reaction Using Enthalpies Of Formation 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.chegg.com
Solved Using The Standard Enthalpies Of Formation Listed Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. to find the δh reaction o, use the formula for the standard enthalpy change of formation: \[\delta h_{reaction}^o = \sum {\delta. 193 rows the standard enthalpy change. Standard Heat Of Reaction Using Enthalpies Of Formation.
From schoolworkhelper.net
StandardEnthalpiesFormation Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. to find. Standard Heat Of Reaction Using Enthalpies Of Formation.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Heat Of Reaction Using Enthalpies Of Formation So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as. \[\delta h_{reaction}^o = \sum {\delta. the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. \[\delta h_{reaction}^o = \sum {\delta. the. Standard Heat Of Reaction Using Enthalpies Of Formation.
From printablelibscapus.z21.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. to find the δh reaction o, use the formula for the. Standard Heat Of Reaction Using Enthalpies Of Formation.
From www.youtube.com
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Standard Heat Of Reaction Using Enthalpies Of Formation a standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. So at this point we have identified three ways. Standard Heat Of Reaction Using Enthalpies Of Formation.